What is the problem?
Some women are at risk for developing blood clots in the legs or lungs (thrombosis) after they deliver a baby (postpartum). A blood clot can form in the lungs , which can be serious. The risk of blood clots is highest in the first 6 weeks after delivery. While we know what the risk factors are for getting a blood clot after delivery, we still don’t know what the best way is to prevent blood clots.
Previous research studies (trials) tried to see if using daily injectable blood thinners after delivery could prevent blood clots. These trials were not successful because taking daily injectable blood thinners at home was not very popular. Many women who deliver babies and have modest risk factors for blood clots may be given injectable blood thinners while they are in hospital, but they usually do not go home on injectable blood thinners.
What is our research study about?
A pilot trial is a smaller trial that is done before a large, expensive trial. One of the goals of our pilot PARTUM trial is to see if we can recruit enough women to show that a larger international trial is possible. Our pilot trial will run across several cities in Canada and in Europe. We expect 384 participants from around the world will be included in our pilot trial.
What is the purpose of our pilot trial?
A pilot trial is a smaller trial that is done before a large, expensive trial. One of the goals of our pilot PARTUM trial is to see if we can recruit enough women to show that a larger international trial is possible. Our pilot trial will run across several cities in Canada and in Europe. We expect 384 participants from around the world will be included in our pilot trial.
In our pilot trial, we will collect information about blood clots and bleeding that can be used in our larger trial. Our larger PARTUM trial will be able to answer if aspirin safely prevents blood clots in postpartum women. The results of the larger trial have the potential to improve the care of postpartum women around the world.
Can I take part in the trial?
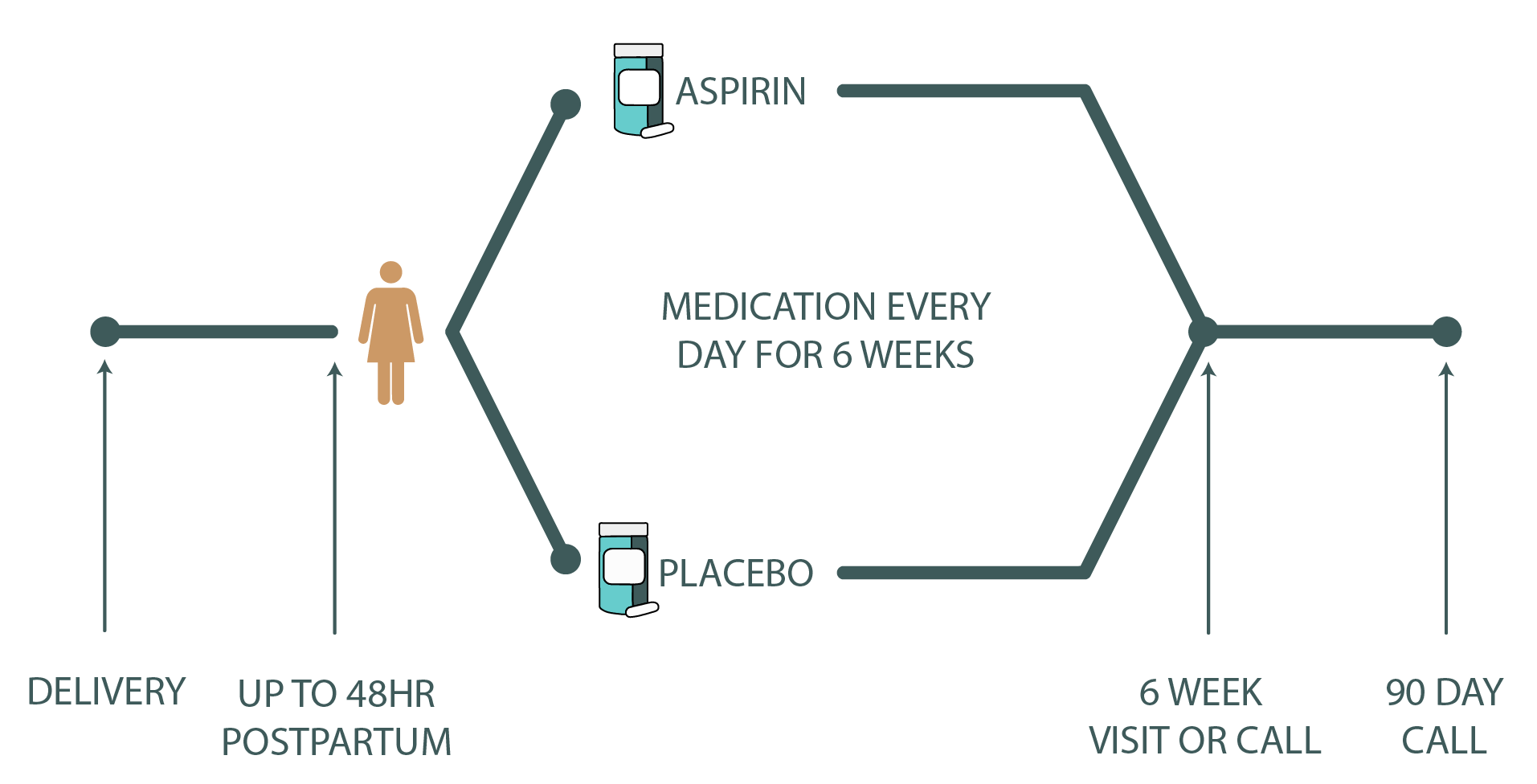
You may be approached about the pilot PARTUM trial if you are pregnant or have given birth within 48 hours and have risk factors for blood clots. Here is a link to our Inclusion Criteria to see if you may be eligible to participate in the pilot PARTUM trial.
If your doctor thinks you are at a high risk for developing blood clots and they recommend that you go home on injectable blood thinners, or you need aspirin for other reasons, then you will not be eligible to participate in this trial.
What is involved in the trial?
If you choose to participate, following the birth of your baby you will be given either low dose aspirin or placebo pills (sugar pill) to take every day for 6 weeks. Neither you nor your doctor will know what treatment you receive (double blinded). A computer system is used to randomly put each participant into either the aspirin or placebo group. Check out our Trial Details to learn more.
Is the pilot PARTUM trial open in my city?
The pilot PARTUM trial will be open for 6 months in each city. Check out our About Us or Contact Us section for more information.