What is the problem we are trying to solve?
Some people are at risk for developing blood clots in the legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) after they deliver a baby (postpartum). These are both serious health conditions and a PE can be life threatening. The risk of blood clots is highest in the first 6 weeks after delivery. While we know the risk factors for getting a blood clot after delivery, we still don’t know the best way to prevent blood clots in the legs and lungs during this time.
Injectable blood thinners called low-molecular-weight-heparin (LMWH) are often given to people who have risk factors for blood clots after delivering their baby to help prevent blood clots. There are not many research studies to guide practice, so there is variation in whether someone gets LMWH injections and for how long after delivery.
Other research has shown that aspirin is safe and works to prevent blood clots in people after hip and knee surgery, which is a high-risk time for getting a blood clot. Aspirin and LMWH are both safe with breastfeeding, and aspirin is a pill instead of an injection.
What is our research study about?
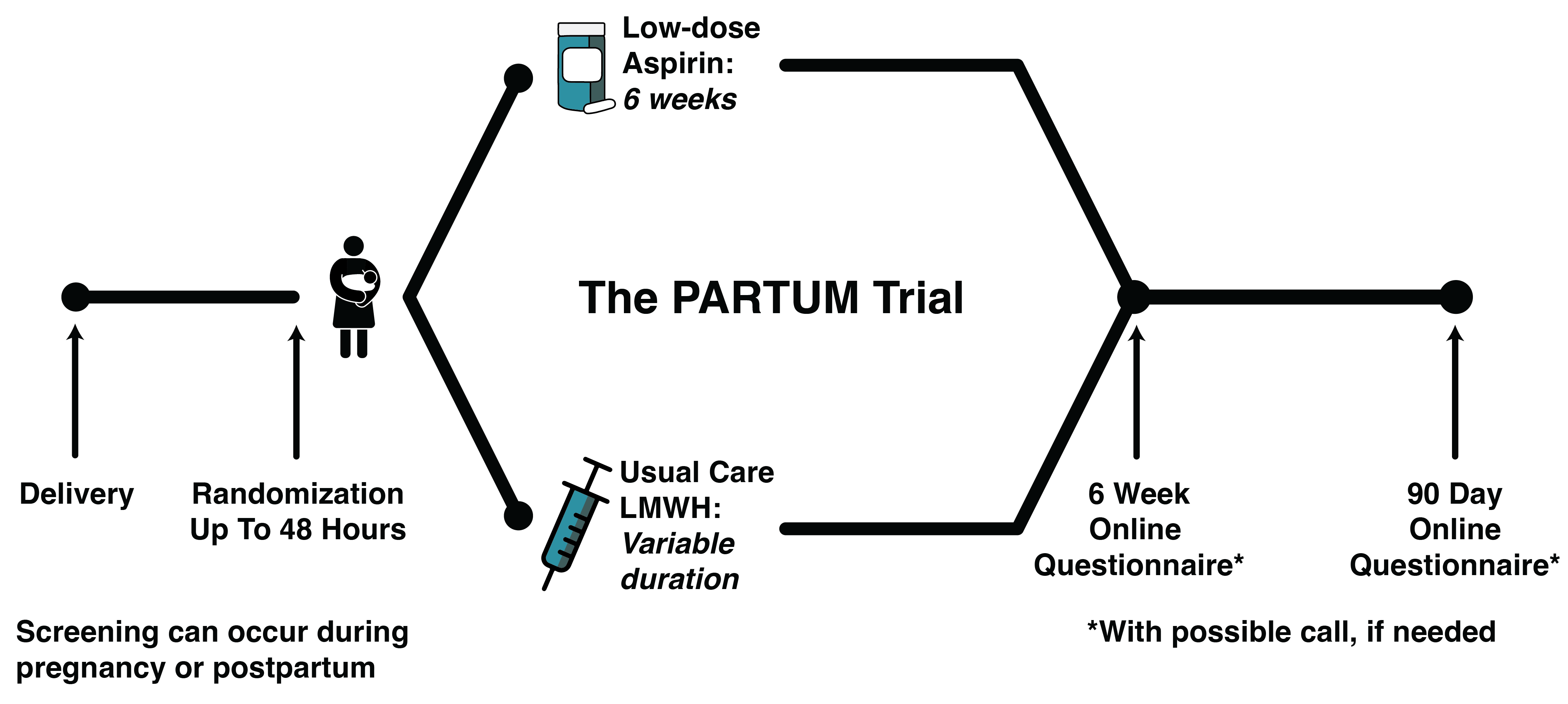
The PARTUM randomized trial asks if taking a daily aspirin postpartum for 6 weeks after delivery is similar to the usual care of taking an injectable blood thinner (LMWH) for the prevention of blood clots. This will allow us to better care for postpartum individuals who are at risk of blood clots.
The PARTUM trial will include 8,805 participants from around the world.
Can I take part in the PARTUM trial?
You may be approached about the PARTUM trial if you are at a participating site and are either pregnant or have given birth within 48 hours and have risk factors for blood clots. Read our Inclusion and Exclusion Criteria page to see if you may be eligible to participate in the PARTUM trial.
If your doctor recommends that you only receive aspirin or LMWH injections because of your medical history, then you will not be eligible to participate in this research study.
What is involved in the trial?
If you choose to take part, following your delivery you will be given either low-dose aspirin (75 mg to 100 mg based on country) every day for 6 weeks (42 days) or you will follow a usual care LMWH daily injection plan.
A computer system is used to randomly put each participant into either the aspirin or usual care group. You, your healthcare team, and the researchers will know which group you are in.
If you are assigned to the aspirin group, you will be given a bottle of the study medication (aspirin) before you leave the hospital. If you are assigned to the usual care LMWH group, you will be given a prescription of LMWH by your doctor. Your doctor will also determine the length of time you will take the daily LMWH injections.
Participation is simple. No blood work, imaging tests or in person appointments are needed in this research study.
Study follow-up will be by a short electronic questionnaire (email or text message) at 6 weeks and 90 days after delivery, and a phone call with a research team member if questions arise, if the questionnaire is not completed, or based on your preference.
Is the PARTUM trial open in my city?
Check out our About Us section or Contact Us for more information.